A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Sunday, January 30, 2011
Hess's Law Lab
Thursday, January 27, 2011
Hess' Law
Once the dry ice melted under the water, it would pop under the pressure and send water everywhere, which was terrifying to some of the people in our class... so this is is the same thing, but without the "pop".
Moving onto the lecture today, we spent the rest of class discussing something called Hess' Law. Hess' Law basically says that you can use multiple ways to find Hess' Law, and it will still work, as long as you come to same, correct conclusion.
"If you ski down a hill, it doesn't matter the path you take, you're still going to get to the bottom. As long as you don't hit a tree."
Basically you are given a formula, and you must add and subtract a series of other equations to match it, and then do the same process to series' delta H's in order to find the original missing delta H. To change the equations you can do one of two things: you can flip the formulas around or multiply their coefficients by a number. When adding the equations, if they are on the opposite side of the arrow and the same element/compound they cancel. If they are on the same side then they combine.
An example:
Wednesday, January 26, 2011
If at Once you don't Succeed Try, Try Again.
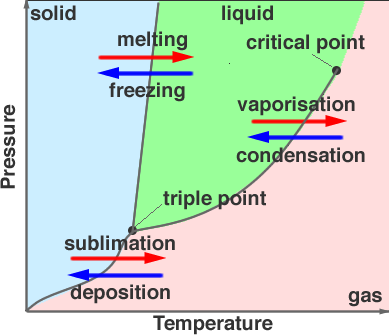
The Phase Diagram above is on Water!!! So the shaded blue part is where at a certain temperature and pressure the water is a Solid (such as Ice). The shaded green part is where the water is a liquid (Aka water). The shaded pink part (sure i guess you can call it peach) is the water as a gas. (The actual H2O molecule, not O2 or H2 molecules) Anyway...the places where the lines separate the three areas are where the change between the phases happen. The temperature is always on the X-axis and the pressure is always on the Y-axis. The lines tell the points at which the change takes place, but there is almost always a 'normal' melting or 'normal' boiling point. Which for water is 0 degrees Celsius and 100 degrees Celsius. The point on the graph that says "Triple Point" is where at this certain pressure and temperature the substance will be in all three states. We actually saw this happen in one of the demo's Mr. Liebs showed us... I couldn't find a picture of the triple point of nitrogen so I'll just use the picture of water...
 As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again.
As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again. We were trying to do the same thing with carbon (aka dry ice) but as I said earlier we couldn't get a good enough seal. Also in the video you will hear the word Viscosity. That means the thickness or thinness of a liquid (also used for oils, if anyone needs to put oil in their cars they look at the viscosity to make sure it's right).
Boiling occurs when the vapor pressure and the pressure from outside are equal, or when the vapor presser exceeds that of the outsides. During the Critical point the meniscus:
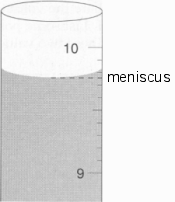
disappears and the point where liquid and gas are separated is nonexistent:

As you can see, the meniscus has disappeared.
Another thing we learned is the term Sublimation, this is when the solid changes to a vapor totally skipping the liquidation phase.
Please don't forget about the two web assigns. One has been extended from Monday, and is now due tomorrow, then also the web assign that is due on the 31st is up. We also have work sheets that are due on the day of the test.
The Scribe for tomorrow is...Erika G.!!!!!!!!! YAY!!!!! Good Luck Erika! And again sorry about the video, I'll figure it out by next time!!!!!
Tuesday, January 25, 2011
Heat

Monday, January 24, 2011
Heating Curve Lab
Title:
Lab Goal:
Data/Collections: (includes data, labeled graph, and percent error)
Conclusion: (includes claim (according to your data...), evidence (what data did you use
that helped you make this claim? How did you identify what ever you were
looking for), and reasoning (does your data make sense? Why might it not be
what you expected it to be?)
In the lab, we filled a 400 mL beaker with about 150 mL of snow/crushed ice. The goal of the lab was to find out the temperature at which ice melts and boils (using the heating curve). The next step was to take the initial temperature of the ice. My group got about -.5º C (however you should turn this number to Kelvin [add 273] because when calculating the percent error it will be difficult to work with Celsius whose freezing point is 0º).
Next, the beaker was placed on a hot plate and the heat was turned on halfway. The crushed ice was stirred and every minute it's temperature was taken. After all the ice melted the hot plate was turned on to full value. We continued to stir the liquid and take its temperature every minute. The hot plate was turned off after 3 minutes of boiling (100º C).
The data you got (temperature and time of the the different phases of the ice) will now be used to make a graph (recall earlier this year when we used excel to make tables and graphs with curves/lines). Instead of writing a post lab, you will write up a conclusion summarizing the lab and explaining at what temperature the ice melted and boiled at and why this is so.
That was about it for today. This lab is due wednesday. Our test will be next thursday (02/03/11). There is a webassign due tomorrow (tuesday the 25), and don't forget to start the second set of book problems which will be due on the day of the test. We will also be turning in the first set of book problems that we did before finals.
The next scribe will be Ann Marie C.
Good luck!
Monday, January 10, 2011
Heat of Formation Lab
Sunday, January 9, 2011
Heat of Formation
http://www.flickr.com/photos/hc1011/5334103360/
http://www.flickr.com/photos/hc1011/5333487119/
http://www.flickr.com/photos/hc1011/5334103324/
http://www.flickr.com/photos/hc1011/5333486983/
After doing the lab, we started learning about Heat of Formation! How exciting. So basically heat of formation is the change in enthalpy that accompanies the formation of one mole of a compound from its elements. Here's a link if you want to learn more about enthalpy! http://en.wikipedia.org/wiki/Enthalpy
By the way, there was no notes sheet about this, so I'll try my best at explaining this. So there's an equation for finding the heat of formation.

So for calculating the Hf (heat of formation), you either have to look it up online, or in the chemistry text book. It's on page 209 or pg 638. Here's a table of what it looks like.

I'm pretty sure Mr. Lieberman said we don't have to memorize it. You only need to use this if it's a compound of two elements. For elements, the heat of formation in its stardard state is 0. Here's an example problem.
Calculate Delta H for reaction Kj/mole. Here's something that you should also consider. The form of the compound (example: gas, liquid, solid) matters.

So first, we find each heat of formation. The heat of formation for NH3 is (-46 kj/mole) You multiply it by 4 because in the equation, there are 4 moles of NH3. Then the heat of formation for O2 is just 0, because oxygen is is in its standard state. Then NO2 is (34 kj/mole) then multiply that by 4. Then finally, H20 is (-286 kj/mole) then multiply that by 6. So then when you multiple it out, the 6 moles of H20=-1716, 4 moles of NO2=136, O2=0, and then 4 moles of NH3=-184. You then take the products-reactants=delta H equation, so (H20+NO2)-(0+ -184)= delta H. Then your answer should equal to -1396. Remember, you always have to do products- reactants, not reactants-products.
After our notes, we had a quick discussion on rare earth metal magnets. To answer Mr. Lieberman's question "do magnets release or absorb energy when pulling them apart, or connecting them together", you have to think about it. Well, you have to use up energy when pulling apart the magnets, and then release energy to combine the magnet. Now think about this. Breaking apart two magnets is like breaking bonds which is an endothermic reaction and combining the magnets is like forming bonds which is an exothermic reaction...

It seems like we'll be learning more about this some other time. Okay, that's it for me. The homework is the pre-lab, webassign, post lab of the heat of combustion, worksheets, AND book problems. Cool. The next scribe is AnnMarie C.
Thursday, January 6, 2011
Endothermic Vs. Exothermic

Delta H stands for the energy released. That is the energy that was released by the reactant to make the product. The H stands for Enthalpy. The Delta H and q have equal values. So Delta H=q. The q stands for hea
Mr. Lieberman showed the class some magnets today and was asking about the energy transfer as they connect and as they detach. What do you think? When put together is the energy absorbed or released, and when detached is the energy absorbed or released? How can you tell? I won't answer, just think about it.
The pre-lab for Heat if Combustion is due tomorrow, also there is a web assign due tomorrow as well. The worksheets are, like always, due on the last day of the chapter, which this time is the day of the quiz (1/11/11).
Wednesday, January 5, 2011
Heat of Fusion of Ice Lab
Next we learned about exothermic and endothermic processes. They both can be chemical reactions or physical changes.
An exothermic process loses eneregy. The energy flows out of the system and to the surroundings. Exothermic processes have a negative q.
An endothermic process gains energy. The energy flows into the system from the surroundings. Endothermic processes have a positive q.
Remember: The q of a system is equal and opposite to the surroundings.
Then we did a short lab.
We had to fill a beaker with about 25g of ice and obtain a beaker with about 100mL of warm water. We recorded the initial temperature of the water. Then we mixed the ice with the water and waited for the ice to melt. Once it had melted we recorded the final temperature of the water. We then used the data we had to find the molar heat of fusion of ice.
In order to find the molar heat of fusion of ice, you have to find the q of the water. Next convert the grams of ice into moles. Then make a ratio (J/mol)
The next scribe is Danielle
Tuesday, January 4, 2011
Specific Heat of Metal Lab
Monday, January 3, 2011
Thermodynamics
First we started talking about two forms of energy, kinetic energy, and potential energy. Kinetic energy is produced with motion. Potential energy is stored energy, and it is produced when not in motion. To relate these two, remember that when kinetic energy goes up, potential energy goes down, and vice versa.
Next we talked about temperature and heat. Temperature is the measurement of the kinetic energy of particles, or how fast the particles are moving. Temperature is measured in degrees of celcius, farenheight, or kelvin. Heat is the measure of energy that transfers from an object of high temperature to an object of low temperature. Heat is measured in units of joules, or the stupid american unit of calories, as Liebs tells us. Remember that heat always transfers from high temperature to low temperature. It never transfers the other way around.
To relate temperature and heat we use heat capacity. Heat capacity is different with every object. It is the amount of heat required to raise the temperature of an object by 1 degree celcius. The formula for heat capacity is:
heat supplied (J) / temperature (C) = heat capacity (J/C)
For the homework we need to figure out how much heat is required to raise an object or a substance by a certain temperature. So use these simple formulas to help you out:
heat required = mass x heat capacity x change in temperature
change in temperature= final temperature - initial temperature
Here is a sample problem:
How much energy is required to increase the temperature of 112 g of water from 35 degrees celcius to 80 degrees celcius, if the heat capacity of water is 4.18 J/gC?
Well this pretty easy, all you do is is find the change in temperature by doing 80-35=45. Then you just plug and chug from here.
energy required = (112 g) x (4.18 J/gC) x (45 C).
so the answer is 21067.2 joules
Well that is all for today. The homework tonight is to finish the Heat Calculations Practice worksheet and maybe review a little for the final if you can. The next scribe is Rachel K.

