A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Tuesday, November 30, 2010
Percent Yield
Monday, November 22, 2010
The Wild Wild World of Limiting Reactants


 .
. is needed to react with the .66 grams of Al.
is needed to react with the .66 grams of Al.



Sunday, November 21, 2010
Limiting Reactants
First we finished our labs by decanting the water of our beakers, and massing our silver. This is largely uneventful, but necessary to complete the lab. The result probably looked something like this:
The post lab gives the conversion 1 Troy ounce= 31.0 grams, so convert your mass to troy ounces, then multiply the times $24.38.
Next Liebs answered questions on the Super Mole Fun worksheet. He has the first three problems worked out here, just scroll down to the super mole fun pdf. (Public Data File)
After that we took our quiz, which covers what Tom talks about in his lovely post below. It was mostly converting grams to moles to grams again, in order to determine how much of a product would be produced, or reactant was need.
Next, were our notes on limiting reactants.
For some reason my computer hates me, and refuses to put the embeded slideshow of the notes in but the link may be found here starting on Slide 8.
A limiting reactant is the reactant that produces the least amount of product. There are two ways to do this. The first is to find to choose one of the actual amounts of reactant used and determine how much of the other reactant it would need. Then compare the number you found to the actual number used, and if it's less than what was used, then that is the limiting reactant. However, Liebs doesn't reccomend this method, instead he suggests the second one: determine in two equations how much of the product each reactant produces, and compare. This method is better because it is clearer, and most labs/worksheets will also ask you how much of the product each reactant produces anyways.
Liebs ended class by tell him two things had made his day: one, was someone couldn't figure out how to turn off a solar-powered calculator. The second was this beauty right here....
"baby monkey, baby monkey..."
Next scribe is..... Amar! Congrats bud.
Thursday, November 18, 2010
We are making silver!!!
 Everyone better watch the Bears own the Dolphins tonight. And the next scribe is...
Everyone better watch the Bears own the Dolphins tonight. And the next scribe is... 
Tuesday, November 16, 2010
A New Hope for Stoichiometry (Chemistry Honors Vs. Chemphys)
Reaction #1: CaC2 + 2H2O --> Ca(OH)2 + C2H2
Then we plugged C2H2 into a combustion reaction: (It is balanced..DUH) 2C2H2 + 5O2 --> 4CO2 + 2H2O
Mr. Lieberman then posed to the question of: If .60 grams of CaC2 reacts, how many grams of H2O will form? ( Use the balanced combustion equation above)
The converstions look like this:
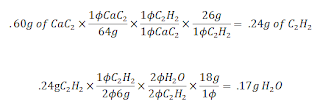
In the end you end up with .17 moles of H2O. Soon after this we witnessed the astonishing demo of Evil KinMOLble! This unlucky or lucky, (I'm not sure what was going through his head..... probably AAAAAAAAAAAAAAAHHHHHHHHHHHHH!!!!!!!!!!!!!) It was the projectilation of Mr. Mole from Mr. Lieberman's Mole Cannon using the combustion. The combustion was caused by the ignition of Acetylene from the reaction of H2O and CaC2(I think not sure on this). After that first demo, we took our short little quiz on Stoichiometry. Lastly, as a conclusion to our class period, Mr. Lieberman armed the launcher once again and fired it into the ChemPhys classroom next door. Causing complete academic Armageddon in the science wing. (ok I exhaggerated a little). Check out the Video in the post underneith this.
The next scribe is Tom btw.
Monday, November 15, 2010
Stoichiometry
Then we got down to business. We went over the notes of the first two slides in Stoich.
Wednesday, November 10, 2010
Honors chem Goes viral
So today we started with a demo that was pretty awesome.
Pb(No3) + KI
Monday, November 8, 2010
Reactions, Reactions, Reactions


Sunday, November 7, 2010
Ionic Equations Galore!
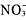 ➝ AgCl + K+ +
➝ AgCl + K+ + 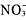
Thursday, November 4, 2010
Solutions and Electrolytes
Reaction 1: 2Mg + O2 ---> 2MgO
Synthesis
Reaction 2: 2HCl + Mg ---> MgCl2 + H2
Single Replacement
Reaction 3: (NH4)2CO3 ---> CO2 + 2NH3 + H20
Decomposition
Reaction 4: CaCO3 + 2HCl ---> CaCl2 + H2O + CO2
Double Replacement
Reaction 5: CuCl2 + Zn ---> ZnCl2 + Cu
Single Replacement
Reaction 6: 3CuCl2 + 2Na3PO4 ---> Cu3(PO4)2 + 6NaCl
Double Replacement
Reaction 7: NaOH + HCl ---> H2O + NaCl
Double Replacement
Next, Mr. Lieberman went over some new notes. We talked about solutions and electrolytes. A Solute is what is being dissolved in a substance (in this case we mainly focused on water). A Solvent is what is doing the dissolving. For example: You have water and salt. Water is the solvent and salt is the solute because it is being dissolved in the water. A solvent can be anything from a liquid, solid, or gas. Water dissociates ionic compounds into its ions (meaning that it takes them apart). Example: NaCl ---> Na+ + Cl-. Another example we used is the rock salt you pour on your driveway and sidewalks in the winter. The formula for it is CaCl2 but when it dissolves in the snow (water) you get Ca (charge of 2+) and 2Cl (charge is -).
If the compound happens to be covalent, the formula stays the same but changes whether it is a gas, solid, or liquid. For example: CO2 (g) ---> CO2 (aq).
Electrolytes are basically ionic compounds that carry a charge. Strong electrolytes are a solution containing enough ions to carry a current efficiently (complete ionization). A weak electrolyte is a compound that doesn't completely dissociate (small amount of ionization).
Electrolytes are essential to our lives. Without them we would die. Athletes drink gatorade because they want those electrolytes. Mr. Lieberman also brought up your nervous system. Neurotransmitters work as ions too. Your nervous system passes electrical currents all the time.
Mr. Lieberman conducted an experiment to show the presence of electrolytes. The goal was to light a lightbulb. At first this was attempted by placing a beaker of salt under the lightbulb where the metal ends were. The lightbulb did not light. Then the same thing was done with sugar. The bulb still did not light. Water was used next but the electrolytes were to weak to light the bulb. When adding a little salt to the water the electrolytes were strengthened and were able to light the bulb. When even more salt was added the electrolytes were even stronger and the light was brighter.
 |
| Strong Electrolytes |
 |
| No Electrolyte |
 |
| Weak Electrolytes |
Wednesday, November 3, 2010
chemical reaction notes and chemical reaction lab
So on Tuesday we finished up the notes and started our classifying chemical reactions lab which we finished today.
There are five types of chemical reactions synthesis, decomposition, single replacement, double replacement, and combustion.
We finished talking about the last three:
3. Single replacement. This type of reaction occurs when there are three elements and one replaces another. Element + compoundà product + product. A + BCà AC + B (if A is a metal) or A + BCà BA + C (if A is a nonmetal).
4. Double replacement. This type of reaction occurs when a metal replaces a metal and a nonmetal and nonmetal. Compound + compound product + product. AB + CD à AD + CB.
5. Combustion. This type of reaction occurs when a hydrocarbon reacts with oxygen gas. In general CxHy + O2 à CO2 + H2O.
Then we did the classifying chemical reactions lab. For this lab you did a series of 7 reactions which each represented 4 out of the 5 reactions (everything but combustion).
So this is what you had to do and the observations me and my partner got:
Reaction #1: You had to heat a piece of magnesium metal and let it light and then hold it over an evaporating dish. Our observations for this were, obviously, that the magnesium light up had a bright glow.
Reaction #2: for this you add hydrochloric acid to a small test tube and then add strip of Magnesium metal. Then you almost immediately light a wood splint and place it in the mouth of a test tube. For this we saw it fizz and make a POP! sound.
Reaction #3: for this you put a piece of ammonium carbonate in a test tube and heat it for 30 seconds. Then you piece of wet litmus paper in the mouth of the test tube and afterwards test the gas with a wood flint. For this we saw that the stick immediately went out after we put it in and the litmus paper turned blue very fast.
Reaction #4: For this reaction we put calcium carbonate in a test tube and added hydrochloric acid to it. Afterwards we light a wood splint and put the burning splint halfway down the test tube. Our observations for this were that there was no temperature change and the splint went out.
Reaction #5: For reaction five you had to add copper (II) chloride solution into a small test tube and add 1 piece of mossy zinc to the test tube. We observed that the mossy zinc instantly turned black.
Reaction #6: For this reaction you had to add copper (II) chloride to a small test tube and add sodium phosphate to that. Our observation for this was that the mixture precipitated and clumped.
Reaction #7: For the final reaction you add sodium hydroxide solution and phenolphthalein into a test tube and mix the solution. Then you add hydrochloric acid until there is a permanent color change. So as you can guess in this reaction we observed a color change. It went from a clear liquid to a pink liquid.
That was all for the lab and class! Tonight’s homework is to finish this lab, it’s due tomorrow.
The next scribe is ……….. Stephanie K.
Monday, November 1, 2010
Balancing Equations and Chemical Reactions
There are five types of chemical reactions. The two we began discussing in class today were:
1. Synthesis reactions. These types of reactions occur when two substances (generally elements) combine and form a compound. Reactant + Reactant --> 1 product or A + B --> AB. An example of a synthesis reaction is 2 H2 + O2 --> 2 H2O
2. Decomposition reactions. This kind of reaction occurs when a compound breaks up into the elements or in a few to simpler compounds. 1 reactant --> product + product or AB --> A + B. An example of this type of reaction is 2 H2O --> 2 H2 + O2
In order to further explain these two reactions, Mr. Lieberman showed us two demonstrations.
http://www.youtube.com/watch?v=8ncN7AdROrY
The first video demonstrates a synthesis reaction. Liebs used a balloon filled with hydrogen gas and a candle as the two reactants. When the open flame touched the candle, it reacted violently with the hydrogen inside the balloon, causing the balloon to pop (although it was more of an explosion) and the product of the reaction was water.
The second video shows an example of a decomposition reaction. A large graduated cylinder was filled with hydrogen peroxide and some dish soap. When Liebs added the catalyst, the hydrogen peroxide reacted and foamed over the top of the graduated cylinder. Liebs calls this demonstration "elephant toothpaste."
http://www.youtube.com/watch?v=f_ftXxCwvDw
So that pretty much sums up what we learned in class today. Sorry everybody, my computer won't let me upload/embed the videos, so I just posted the web addresses to each of the videos. Hopefully they work.
Also, the next scribe is................. Nirali P.









