A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Wednesday, March 23, 2011
Colligative Properties Lab
T(b)=i x m x k(b)
You are supposed to solve for molality here. The ionic solid has 2 ions so i would equal 2. m would equal the molality and k(b) is a constant, which would be 0.51 for water. The T(b) is the Delta H that comes from the data in the lab. The homework is to finish up the post lab which is due on friday. Also there are 3 WebAssigns due on friday so keep up with them. The next scribe is Lauren C.
Tuesday, March 22, 2011
Colligative Properties
1.Vapor Pressure Lowering
2. Boiling Point Elevation
3. Melting Point Depression
4. Osmotic Pressure
Basically, the relations are simple. The more solute added, the more the vapor pressure decreases. The more solute added, the more the boiling point raises. The more solute added, the lower the melting point becomes. We also got a few formulas to accompany the above.
To calculate the change in boiling point(Blogger isn't letting me use equation editor, get notes off moodle to really see this):
Change in Boiling Point=molal boiling point elevation constant x molality
Traingle T subscript b= K subscript b * m
To calculate freezing point depression, you use the same formula except the constant is different. The K subscript b is repaced with K subscript f.
The van't Hoff factor basically mens that if you have a mole of NaCl, and the ions dissociate when in water, you will get one mole of Na+ ions and one mole of Cl- ions. The van't Hoff factor is i so simply modify the previous equations by multiply i to the end like so:
Triangle T subscript f= K subscript f * m * i
That pretty much sums it up for new material covered today. Liebs demonstrated these new concepts with one demo involving 4 of us as water molecules, showing that the more solute added (balloons), the more interactions we had to make with it (touching each ion), which made it harder for us water molecules to turn into our gaseous state. Also, we took some club soda, stuck it into some ice and upon opening the glass, thus releasing the CO2 which had raised the freezing point, the club soda immediately froze.
Liebs also discussed some issues with the faulty Webassign that was due today, and said he would make corrections. I know it probably frustated myself as much as did to some of you (honestly, comment if you remembered how to use sig figs before this unit started).
That's about all though. Homework tonight is to do all the worksheets you have been given this unit that are due on the day of the test, as well as the 3 remaining webassigns also due on the day of the test.
Justin J., come on down, you are the next contestant on the price is right.
Monday, March 21, 2011
Stoichiometry Returns


Sunday, March 20, 2011
Hey Guys! So today in chem class we went over notes on molarity and molality dilution.
The first concept that we talked about was the concentration of solute. That is, the amount of solute in a solution is given by its concentration. There are four units if concentration.
The first is molartiy and you can compute it by this equation, molarity (M) = moles of solute/liters of solution. This equation comes in hand when you are trying to figure out the mass of a substance needed to make a certain amount of solution at a specific molarity, or to simply find the molarity of a solution.
The second unit in with you can compute the concentration of a solution is molality, be sure to not get this and molarity confused for the sound very similar. With this unit the equation is molality (m) of solution moles of solute/kilograms of solvent.
The third unit in with you can compute the concentration of a solution is percent by mass. The equation of this unit is % by mass = (grams of solute/grams of solution) X 100.
The fourth and final way of compute the concentration of a solution is by finding the mole fraction. To find this you use the equation mole fraction (x) = moles of solute/ moles of solvent + moles of solution. For this you want to remember to have your final answer be in decimals.
The second concept we discussed was molarity and dilution. This concept talked about diluting a solution and this is simply adding more solvent, in our case water, to the solution. This will dilute the solution but remember that the amount of solute does not change and because of this, it is really easy to calculate the new molarity. All you do is take the information you have (at least three points) and plug it into the equation, MaVa=MbVb.
That was all for today’s class. Homework for Monday is to finish your lab and do the webassigns also remember to keep up with your work sheets.
The next scribe is …………………….. Rachel M.
Thursday, March 17, 2011
Solubility Curve Lab

Today in 7th Period Chem we did yet another lab! This one dealt with measuring the saturation temperatures for six difference solution concentrates to construct a solubility curve.
Wednesday, March 16, 2011
Baby Monkey Going Backwards on a Pig


Tuesday, March 15, 2011
Solution Formation Lab

In the shortened period today we did the Solution Formation Lab. The goal of the lab was to determine the effect of three variables on the solubility of copper sulfate in water. These variables were: crystal size, temperature, and degree of mixing. Based on my group's (Trevor B, Artie B, and myself...decide if you trust us or not) data, we came to the conclusion copper sulfate was most soluble under the following conditions:
- higher temperature
- smaller crystals
- and vigorous stirring
Conversely, the least soluble conditions are:
- lower temperature
- larger crystals
- and little to no stirring
And that's about it for today. Work on your Webassigns and the lab writeup is due Thursday. Until we meet again,
Monday, March 14, 2011
Solution Chemistry Day 1
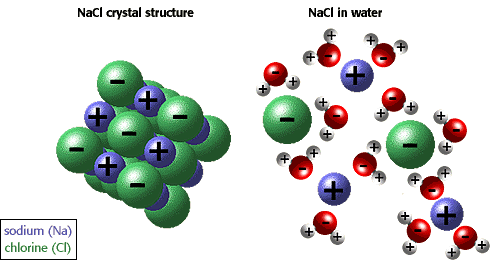
Wednesday, March 9, 2011
Intermolecular Forces


Non-Polar Vs Polar Molecules


Monday, March 7, 2011
Polarity
Building the Big Ones




Wednesday, March 2, 2011
VESPER 2
Tuesday, March 1, 2011
Valence Shell Electron Pair Repulsion, and how it affects the structure of Atoms.
1.The first of these are Bonds.
2.The second are Non-Bonding pairs.
Both of these will affect the Structure of our molecule, but the only part of the structure we see are the bonded Pairs. This will make more sense whence you understand the Valence Shell Electron Pair Repulsion (VSEPR) rule. Under VSEPR electrons pairs in the outermost shells will attempt to go as far away from each other as possible. This means that although we don't look at the Non-Bonding electrons in the structure, they still are Under VSEPR and are effecting the shape of the electrons.
Although it is called Electron Pair Repulsion, we are not simply looking at the pairs, we are looking at areas of electron. I mean to say that if we were to encounter a double bond between two atoms, we would not expect the two pairs involved in the bonding to repel each other. No, these Double Bonds and triple bonds stick together.
In drawing the shape of Atoms we look at the number of Electron Areas around the Central Atom. A different structure is given based on how many electron Area's are around the central Atom and how many of these Areas are Bonds or Non-bonding Pairs. The Notes did a very good job of going through these types but because I do not have access to them, I'll go through it with you now.
Linear is the structure given when their are only two Electron Areas surrounding the central atom and both are Bonds. The atoms are exactly 180 degrees from each other. This structure looks like this
Trigonal Planar this structure happens when the central atom has three electron Areas and all three are Bonds. The atoms still all fit on a plane and are all 120 degrees from eachother. This structure looks like so
Bent Molecule This happens when their are only two Bonds, like Linear, but the central atom also has A non-bounding pair. This causes the atoms to remain 120 degrees from eachother but we only see two of the atoms, and we see a bent structure like this
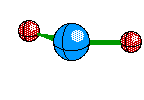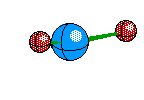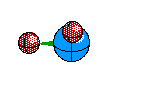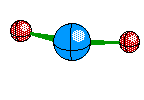
Tetrahedral Molecules get a little more complex because they now become three dimensional. When their are four electron areas, the atom seperates every atom by 109.5 degrees to look like so
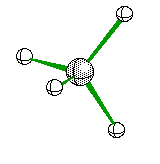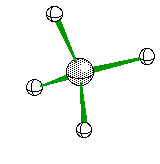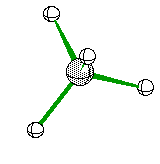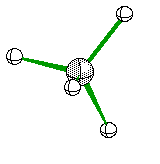
or when drawn on paper it looks something like
Triangular Pyramid when one of the four electron areas is a non-bonding pair it looks something like this
If two of the four electron ares are Non-Bonding then we see another Bent Structure, but in this case it is slightly more tight of an angle creating about a 104 degree bent.
Well there you have the atom structures that we have learned so far. No homework tonight but try to keep on track of upcoming webassigns. thank you, the next scribe will be
Takashi, have fun.
Where are all my electrons?


A double headed Arrow separates the two lewis structures letting us know that if we were to take a snapshot of the atom at any given time, it would look like one of these two diagrams.
One other point brought up in class today was Ions. Ions are slightly more complex to draw in a Lewis, structure, but fortunately not too complex. Their are two main steps that are need to remember.
1. The first is that the molecule is not neutral and therefore has either more or less electrons. If the Ion has a charge of 2-, then it has two extra valence electrons.
2. The second difference between these and neutral molecules is that you must bracket in your entire Lewis structure and write the charge of the Ion outside of the brackets at the top right, as you would for a power of.
To put both lessons together, the following shows the Resonance structure of the Ion NO3(-)
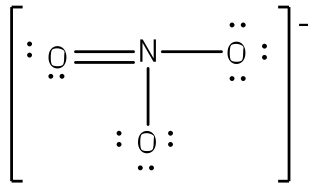 | 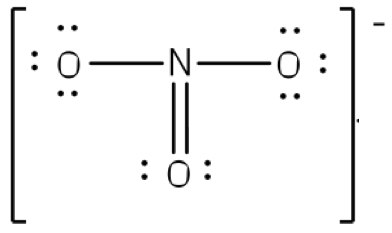 |  |
Thursday, February 24, 2011
Covalent Bonding
 F2 is a very simple example of a covalent bond. They share electrons with each other.
F2 is a very simple example of a covalent bond. They share electrons with each other. 
Ionic Bonding
Thursday, February 17, 2011
Periodicity
Today during class we discussed the trends that have recently appeared in the organization of the Periodic Table. Using the graphs we created the previous day, we looked at Ionization Energy and Atomic Radius in relation to the elements, and their organization in periods and groups.
1. First Ionization Energy (the difficulty of losing one electron--first level)
--As you move top to bottom in groups, it is easier to lose electrons, therefore the first ionization energy decreases. This is because as you move further down, there are more levels of electron orbitals, and they are easier to loose the farther they are from the nucleus
--As you move left to right in periods, it becomes harder to lose electrons, therfore the first ionization energy increases. This is because as you move across left to right the atomic number increases therefore so do the number of protons, which means there is greater pull on the electrons you are trying to lose.
2. Atomic Radius (size)
--As you move top to bottom in groups, the atomic radii increase. This is because there are more levels surrounding the nucleus, therefore larger atoms
--As you move left to right across the periods, the atomic radii decreases. This is because as you move across periods, the atomic numbers increase, and therefore so do the number of protons in the atom. The greater number of protons, the larger pull on the electron orbitals. The greater the pull on the orbitals, the closer they will be pulled into the nucleus, in turn the atoms will be smaller.
Next Scribe...
Rachel Mann
Tuesday, February 15, 2011
orbital diagrams
Saturday, February 12, 2011
Electron Configurations
- The elements in groups 1 and 2 on the Periodic Table are filling an s sublevel. Thus, Li and Be in the second period fill the 2s sublevel. Na and Mg in the third period fill the 3s sublevel and so on.
- The elements in groups 13 through 18 (six elements in each period) fill p sublevels, which have a capacity of six electrons. In the second period, the 2p sublevel starts to fill with B and is completed with Ne. In the third period, the elements Al through Ar fill the 3p sublevel.
- The transition metals, in the center of the periodic table, fill d sublevels. Remember that a d sublevel can hold ten electrons. In the fourth period, the ten elements Sc through Zn fill the 3d sublevel. In the fifth period, the 4d sublevel is filled by the elements Y through Cd. The ten transition metals in the sixth period fill the 5d sublevel. Elements 103 to 112 in the seventh period are believed to be filling the 6d sublevel.
- The two sets of 14 elements listed separately at the bottom of the table are filling f siblevels with a principle quantum number two less than the period number. That is... 14 elements in the sixth period (elements 57 to 70) are filling the 4f sublevel.

An example of an equation we did in class is as follows:
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5p^2 6s^2 4f^14 5d^10 6p^6
The subscripts tell you the energy level, the letters tell you the orbitals, and the exponents (or superscripts), when added together, tell you the electrons. If you were to add all the exponents in this equation, the number would be 86, which is the element Rn (radon).
The next scribe is...
Matt B.
Quantum Numbers
 On Thursday we got some new class notes. We talked about the Quantum Mechanical Model and Quantum Numbers.
On Thursday we got some new class notes. We talked about the Quantum Mechanical Model and Quantum Numbers.- They are used to specify the "address" of each electron in an atom.
- No atom has the exact same quantum number as another atom, they are all unique (refer to the stadium model in the notes).
- There are four quantum numbers:
Principal Quantum Number (n) which is the most general #. It tells us the energy level and sixe of the orbital. Note: These numbers can only have integral values, and the must be positive.
Angular Momentum Quantum # (l) which tells us the enegry sublevel, type of orbital, and shape of orbital (s, p, d, or f). The value of l has integral values from 0 to n-1, and is related to the shape of the orbital. l=0 is s orbital, l=1 is p orbital, l=2 is d orbital, l=3 is f orbital.
Magnetic Quantum Number (ml) tells us the orientation of the orbital, specifies the exact orbital within each sublevel, and has values between l and -l.
Spin Quantum Number (ms) has an electron spin of either -1/2 or +1/2. An orbital can hold two electrons as long as they are spinning in opposite directions. 
That pretty much covers what we learned on Thursday. The next scribe is me again, since I forgot to scribe today.
Wednesday, February 9, 2011
Light

The types of light on the left side of the electro magnetic spectrum have a high frequency and small wave length. The types of light on the right side have a low frequency and a large wavelength.
We then took out our cell phones and looked at the infrared light given off through the remote.
Wednesday, February 2, 2011
Tuesday, February 1, 2011
Spontaneous reactions, Entropy and Gibb
Sunday, January 30, 2011
Hess's Law Lab
Thursday, January 27, 2011
Hess' Law
Once the dry ice melted under the water, it would pop under the pressure and send water everywhere, which was terrifying to some of the people in our class... so this is is the same thing, but without the "pop".
Moving onto the lecture today, we spent the rest of class discussing something called Hess' Law. Hess' Law basically says that you can use multiple ways to find Hess' Law, and it will still work, as long as you come to same, correct conclusion.
"If you ski down a hill, it doesn't matter the path you take, you're still going to get to the bottom. As long as you don't hit a tree."
Basically you are given a formula, and you must add and subtract a series of other equations to match it, and then do the same process to series' delta H's in order to find the original missing delta H. To change the equations you can do one of two things: you can flip the formulas around or multiply their coefficients by a number. When adding the equations, if they are on the opposite side of the arrow and the same element/compound they cancel. If they are on the same side then they combine.
An example:
Wednesday, January 26, 2011
If at Once you don't Succeed Try, Try Again.
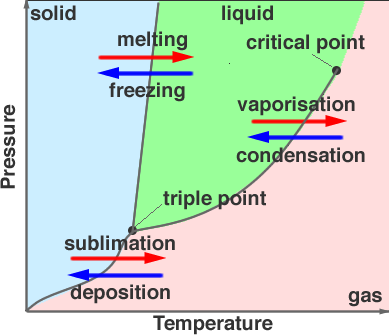
The Phase Diagram above is on Water!!! So the shaded blue part is where at a certain temperature and pressure the water is a Solid (such as Ice). The shaded green part is where the water is a liquid (Aka water). The shaded pink part (sure i guess you can call it peach) is the water as a gas. (The actual H2O molecule, not O2 or H2 molecules) Anyway...the places where the lines separate the three areas are where the change between the phases happen. The temperature is always on the X-axis and the pressure is always on the Y-axis. The lines tell the points at which the change takes place, but there is almost always a 'normal' melting or 'normal' boiling point. Which for water is 0 degrees Celsius and 100 degrees Celsius. The point on the graph that says "Triple Point" is where at this certain pressure and temperature the substance will be in all three states. We actually saw this happen in one of the demo's Mr. Liebs showed us... I couldn't find a picture of the triple point of nitrogen so I'll just use the picture of water...
 As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again.
As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again. We were trying to do the same thing with carbon (aka dry ice) but as I said earlier we couldn't get a good enough seal. Also in the video you will hear the word Viscosity. That means the thickness or thinness of a liquid (also used for oils, if anyone needs to put oil in their cars they look at the viscosity to make sure it's right).
Boiling occurs when the vapor pressure and the pressure from outside are equal, or when the vapor presser exceeds that of the outsides. During the Critical point the meniscus:
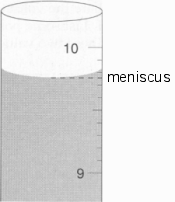
disappears and the point where liquid and gas are separated is nonexistent:

As you can see, the meniscus has disappeared.
Another thing we learned is the term Sublimation, this is when the solid changes to a vapor totally skipping the liquidation phase.
Please don't forget about the two web assigns. One has been extended from Monday, and is now due tomorrow, then also the web assign that is due on the 31st is up. We also have work sheets that are due on the day of the test.
The Scribe for tomorrow is...Erika G.!!!!!!!!! YAY!!!!! Good Luck Erika! And again sorry about the video, I'll figure it out by next time!!!!!
Tuesday, January 25, 2011
Heat

Monday, January 24, 2011
Heating Curve Lab
Title:
Lab Goal:
Data/Collections: (includes data, labeled graph, and percent error)
Conclusion: (includes claim (according to your data...), evidence (what data did you use
that helped you make this claim? How did you identify what ever you were
looking for), and reasoning (does your data make sense? Why might it not be
what you expected it to be?)
In the lab, we filled a 400 mL beaker with about 150 mL of snow/crushed ice. The goal of the lab was to find out the temperature at which ice melts and boils (using the heating curve). The next step was to take the initial temperature of the ice. My group got about -.5º C (however you should turn this number to Kelvin [add 273] because when calculating the percent error it will be difficult to work with Celsius whose freezing point is 0º).
Next, the beaker was placed on a hot plate and the heat was turned on halfway. The crushed ice was stirred and every minute it's temperature was taken. After all the ice melted the hot plate was turned on to full value. We continued to stir the liquid and take its temperature every minute. The hot plate was turned off after 3 minutes of boiling (100º C).
The data you got (temperature and time of the the different phases of the ice) will now be used to make a graph (recall earlier this year when we used excel to make tables and graphs with curves/lines). Instead of writing a post lab, you will write up a conclusion summarizing the lab and explaining at what temperature the ice melted and boiled at and why this is so.
That was about it for today. This lab is due wednesday. Our test will be next thursday (02/03/11). There is a webassign due tomorrow (tuesday the 25), and don't forget to start the second set of book problems which will be due on the day of the test. We will also be turning in the first set of book problems that we did before finals.
The next scribe will be Ann Marie C.
Good luck!
Monday, January 10, 2011
Heat of Formation Lab
Sunday, January 9, 2011
Heat of Formation
http://www.flickr.com/photos/hc1011/5334103360/
http://www.flickr.com/photos/hc1011/5333487119/
http://www.flickr.com/photos/hc1011/5334103324/
http://www.flickr.com/photos/hc1011/5333486983/
After doing the lab, we started learning about Heat of Formation! How exciting. So basically heat of formation is the change in enthalpy that accompanies the formation of one mole of a compound from its elements. Here's a link if you want to learn more about enthalpy! http://en.wikipedia.org/wiki/Enthalpy
By the way, there was no notes sheet about this, so I'll try my best at explaining this. So there's an equation for finding the heat of formation.

So for calculating the Hf (heat of formation), you either have to look it up online, or in the chemistry text book. It's on page 209 or pg 638. Here's a table of what it looks like.

I'm pretty sure Mr. Lieberman said we don't have to memorize it. You only need to use this if it's a compound of two elements. For elements, the heat of formation in its stardard state is 0. Here's an example problem.
Calculate Delta H for reaction Kj/mole. Here's something that you should also consider. The form of the compound (example: gas, liquid, solid) matters.

So first, we find each heat of formation. The heat of formation for NH3 is (-46 kj/mole) You multiply it by 4 because in the equation, there are 4 moles of NH3. Then the heat of formation for O2 is just 0, because oxygen is is in its standard state. Then NO2 is (34 kj/mole) then multiply that by 4. Then finally, H20 is (-286 kj/mole) then multiply that by 6. So then when you multiple it out, the 6 moles of H20=-1716, 4 moles of NO2=136, O2=0, and then 4 moles of NH3=-184. You then take the products-reactants=delta H equation, so (H20+NO2)-(0+ -184)= delta H. Then your answer should equal to -1396. Remember, you always have to do products- reactants, not reactants-products.
After our notes, we had a quick discussion on rare earth metal magnets. To answer Mr. Lieberman's question "do magnets release or absorb energy when pulling them apart, or connecting them together", you have to think about it. Well, you have to use up energy when pulling apart the magnets, and then release energy to combine the magnet. Now think about this. Breaking apart two magnets is like breaking bonds which is an endothermic reaction and combining the magnets is like forming bonds which is an exothermic reaction...

It seems like we'll be learning more about this some other time. Okay, that's it for me. The homework is the pre-lab, webassign, post lab of the heat of combustion, worksheets, AND book problems. Cool. The next scribe is AnnMarie C.
Thursday, January 6, 2011
Endothermic Vs. Exothermic

Delta H stands for the energy released. That is the energy that was released by the reactant to make the product. The H stands for Enthalpy. The Delta H and q have equal values. So Delta H=q. The q stands for hea
Mr. Lieberman showed the class some magnets today and was asking about the energy transfer as they connect and as they detach. What do you think? When put together is the energy absorbed or released, and when detached is the energy absorbed or released? How can you tell? I won't answer, just think about it.
The pre-lab for Heat if Combustion is due tomorrow, also there is a web assign due tomorrow as well. The worksheets are, like always, due on the last day of the chapter, which this time is the day of the quiz (1/11/11).




