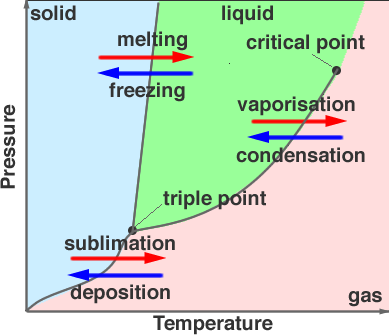
The Phase Diagram above is on Water!!! So the shaded blue part is where at a certain temperature and pressure the water is a Solid (such as Ice). The shaded green part is where the water is a liquid (Aka water). The shaded pink part (sure i guess you can call it peach) is the water as a gas. (The actual H2O molecule, not O2 or H2 molecules) Anyway...the places where the lines separate the three areas are where the change between the phases happen. The temperature is always on the X-axis and the pressure is always on the Y-axis. The lines tell the points at which the change takes place, but there is almost always a 'normal' melting or 'normal' boiling point. Which for water is 0 degrees Celsius and 100 degrees Celsius. The point on the graph that says "Triple Point" is where at this certain pressure and temperature the substance will be in all three states. We actually saw this happen in one of the demo's Mr. Liebs showed us... I couldn't find a picture of the triple point of nitrogen so I'll just use the picture of water...
 As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again.
As you can see, the Liquid is on the bottom, the Solid is in the center and the Gas is on the top. (trust me the gas is there). The critical point is where the liquid is not a solid, liquid, or gas actually. I know it's hard to believe...but it's true. Liebs tried to show us that it was true with dry ice, but we couldn't get a good enough seal on the container so it didn't work. We tried to seal it many, many times but in the end all we had was a broken ego and a wish to see the critical point. So as you can see, 'if at once you don't succeed try, try again.' doesn't always work. But being as optimistic as we are, we will try it again tomorrow. So either it will work, or we will fail...again. We were trying to do the same thing with carbon (aka dry ice) but as I said earlier we couldn't get a good enough seal. Also in the video you will hear the word Viscosity. That means the thickness or thinness of a liquid (also used for oils, if anyone needs to put oil in their cars they look at the viscosity to make sure it's right).
Boiling occurs when the vapor pressure and the pressure from outside are equal, or when the vapor presser exceeds that of the outsides. During the Critical point the meniscus:
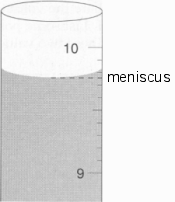
disappears and the point where liquid and gas are separated is nonexistent:

As you can see, the meniscus has disappeared.
Another thing we learned is the term Sublimation, this is when the solid changes to a vapor totally skipping the liquidation phase.
Please don't forget about the two web assigns. One has been extended from Monday, and is now due tomorrow, then also the web assign that is due on the 31st is up. We also have work sheets that are due on the day of the test.
The Scribe for tomorrow is...Erika G.!!!!!!!!! YAY!!!!! Good Luck Erika! And again sorry about the video, I'll figure it out by next time!!!!!
No comments:
Post a Comment