A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Wednesday, March 23, 2011
Colligative Properties Lab
T(b)=i x m x k(b)
You are supposed to solve for molality here. The ionic solid has 2 ions so i would equal 2. m would equal the molality and k(b) is a constant, which would be 0.51 for water. The T(b) is the Delta H that comes from the data in the lab. The homework is to finish up the post lab which is due on friday. Also there are 3 WebAssigns due on friday so keep up with them. The next scribe is Lauren C.
Tuesday, March 22, 2011
Colligative Properties
1.Vapor Pressure Lowering
2. Boiling Point Elevation
3. Melting Point Depression
4. Osmotic Pressure
Basically, the relations are simple. The more solute added, the more the vapor pressure decreases. The more solute added, the more the boiling point raises. The more solute added, the lower the melting point becomes. We also got a few formulas to accompany the above.
To calculate the change in boiling point(Blogger isn't letting me use equation editor, get notes off moodle to really see this):
Change in Boiling Point=molal boiling point elevation constant x molality
Traingle T subscript b= K subscript b * m
To calculate freezing point depression, you use the same formula except the constant is different. The K subscript b is repaced with K subscript f.
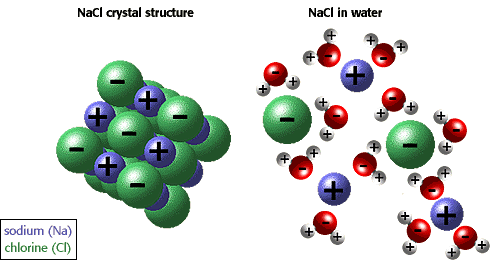
The van't Hoff factor basically mens that if you have a mole of NaCl, and the ions dissociate when in water, you will get one mole of Na+ ions and one mole of Cl- ions. The van't Hoff factor is i so simply modify the previous equations by multiply i to the end like so:
Triangle T subscript f= K subscript f * m * i
That pretty much sums it up for new material covered today. Liebs demonstrated these new concepts with one demo involving 4 of us as water molecules, showing that the more solute added (balloons), the more interactions we had to make with it (touching each ion), which made it harder for us water molecules to turn into our gaseous state. Also, we took some club soda, stuck it into some ice and upon opening the glass, thus releasing the CO2 which had raised the freezing point, the club soda immediately froze.
Liebs also discussed some issues with the faulty Webassign that was due today, and said he would make corrections. I know it probably frustated myself as much as did to some of you (honestly, comment if you remembered how to use sig figs before this unit started).
That's about all though. Homework tonight is to do all the worksheets you have been given this unit that are due on the day of the test, as well as the 3 remaining webassigns also due on the day of the test.
Justin J., come on down, you are the next contestant on the price is right.
Monday, March 21, 2011
Stoichiometry Returns


Sunday, March 20, 2011
Hey Guys! So today in chem class we went over notes on molarity and molality dilution.
The first concept that we talked about was the concentration of solute. That is, the amount of solute in a solution is given by its concentration. There are four units if concentration.
The first is molartiy and you can compute it by this equation, molarity (M) = moles of solute/liters of solution. This equation comes in hand when you are trying to figure out the mass of a substance needed to make a certain amount of solution at a specific molarity, or to simply find the molarity of a solution.
The second unit in with you can compute the concentration of a solution is molality, be sure to not get this and molarity confused for the sound very similar. With this unit the equation is molality (m) of solution moles of solute/kilograms of solvent.
The third unit in with you can compute the concentration of a solution is percent by mass. The equation of this unit is % by mass = (grams of solute/grams of solution) X 100.
The fourth and final way of compute the concentration of a solution is by finding the mole fraction. To find this you use the equation mole fraction (x) = moles of solute/ moles of solvent + moles of solution. For this you want to remember to have your final answer be in decimals.
The second concept we discussed was molarity and dilution. This concept talked about diluting a solution and this is simply adding more solvent, in our case water, to the solution. This will dilute the solution but remember that the amount of solute does not change and because of this, it is really easy to calculate the new molarity. All you do is take the information you have (at least three points) and plug it into the equation, MaVa=MbVb.
That was all for today’s class. Homework for Monday is to finish your lab and do the webassigns also remember to keep up with your work sheets.
The next scribe is …………………….. Rachel M.
Thursday, March 17, 2011
Solubility Curve Lab

Today in 7th Period Chem we did yet another lab! This one dealt with measuring the saturation temperatures for six difference solution concentrates to construct a solubility curve.
Wednesday, March 16, 2011
Baby Monkey Going Backwards on a Pig


Tuesday, March 15, 2011
Solution Formation Lab

In the shortened period today we did the Solution Formation Lab. The goal of the lab was to determine the effect of three variables on the solubility of copper sulfate in water. These variables were: crystal size, temperature, and degree of mixing. Based on my group's (Trevor B, Artie B, and myself...decide if you trust us or not) data, we came to the conclusion copper sulfate was most soluble under the following conditions:
- higher temperature
- smaller crystals
- and vigorous stirring
Conversely, the least soluble conditions are:
- lower temperature
- larger crystals
- and little to no stirring
And that's about it for today. Work on your Webassigns and the lab writeup is due Thursday. Until we meet again,
Monday, March 14, 2011
Solution Chemistry Day 1
Wednesday, March 9, 2011
Intermolecular Forces


Non-Polar Vs Polar Molecules


Monday, March 7, 2011
Polarity
Building the Big Ones




Wednesday, March 2, 2011
VESPER 2
Tuesday, March 1, 2011
Valence Shell Electron Pair Repulsion, and how it affects the structure of Atoms.
1.The first of these are Bonds.
2.The second are Non-Bonding pairs.
Both of these will affect the Structure of our molecule, but the only part of the structure we see are the bonded Pairs. This will make more sense whence you understand the Valence Shell Electron Pair Repulsion (VSEPR) rule. Under VSEPR electrons pairs in the outermost shells will attempt to go as far away from each other as possible. This means that although we don't look at the Non-Bonding electrons in the structure, they still are Under VSEPR and are effecting the shape of the electrons.
Although it is called Electron Pair Repulsion, we are not simply looking at the pairs, we are looking at areas of electron. I mean to say that if we were to encounter a double bond between two atoms, we would not expect the two pairs involved in the bonding to repel each other. No, these Double Bonds and triple bonds stick together.
In drawing the shape of Atoms we look at the number of Electron Areas around the Central Atom. A different structure is given based on how many electron Area's are around the central Atom and how many of these Areas are Bonds or Non-bonding Pairs. The Notes did a very good job of going through these types but because I do not have access to them, I'll go through it with you now.
Linear is the structure given when their are only two Electron Areas surrounding the central atom and both are Bonds. The atoms are exactly 180 degrees from each other. This structure looks like this
Trigonal Planar this structure happens when the central atom has three electron Areas and all three are Bonds. The atoms still all fit on a plane and are all 120 degrees from eachother. This structure looks like so
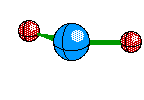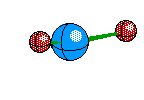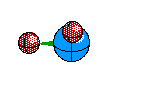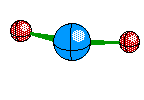
Bent Molecule This happens when their are only two Bonds, like Linear, but the central atom also has A non-bounding pair. This causes the atoms to remain 120 degrees from eachother but we only see two of the atoms, and we see a bent structure like this
Tetrahedral Molecules get a little more complex because they now become three dimensional. When their are four electron areas, the atom seperates every atom by 109.5 degrees to look like so
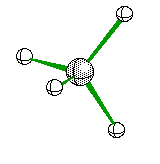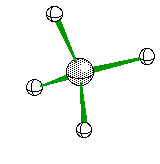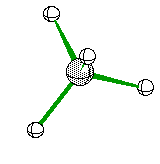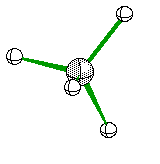
or when drawn on paper it looks something like
Triangular Pyramid when one of the four electron areas is a non-bonding pair it looks something like this
If two of the four electron ares are Non-Bonding then we see another Bent Structure, but in this case it is slightly more tight of an angle creating about a 104 degree bent.
Well there you have the atom structures that we have learned so far. No homework tonight but try to keep on track of upcoming webassigns. thank you, the next scribe will be
Takashi, have fun.
Where are all my electrons?


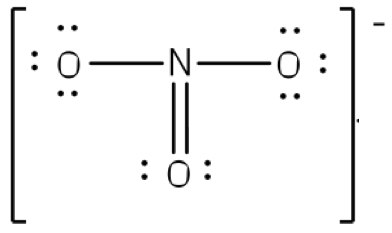
A double headed Arrow separates the two lewis structures letting us know that if we were to take a snapshot of the atom at any given time, it would look like one of these two diagrams.
One other point brought up in class today was Ions. Ions are slightly more complex to draw in a Lewis, structure, but fortunately not too complex. Their are two main steps that are need to remember.
1. The first is that the molecule is not neutral and therefore has either more or less electrons. If the Ion has a charge of 2-, then it has two extra valence electrons.
2. The second difference between these and neutral molecules is that you must bracket in your entire Lewis structure and write the charge of the Ion outside of the brackets at the top right, as you would for a power of.
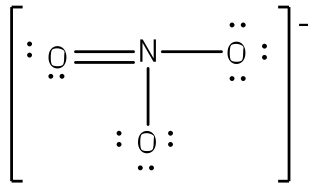
To put both lessons together, the following shows the Resonance structure of the Ion NO3(-)
 |  |  |
