Today we started our new unit on Solution Chemistry. He handed out a new calendar and a new objective sheet. He then gave us the new notes (not online anywhere). We began the period by looking over tests that we took last week. We made another mighty fine discussion and some good laughs courtesy of the usual people. We began our notes discussion with the definitions:
Solution: a homogeneous mixture (all one colored M&M's)
Solute: is dissolved in the solvent
Saturated solution: is one where the concentration is at a maximum, no more solute is able to dissolve.
Supersaturated: a solution that is made at a higher temperature, and when it cools there is more solute than if it were only saturated.
Mr. Liebs then did a sweet demo of how H2O molecules would break dissolve a Salt (NaCl). The two substances would go through three process's before they would become a solution.
-Separation of the solute: the molecules overcome their IMF, using energy (endothermic)
-Separation of the Solvent: the molecules overcome their IMF, using energy (endo).
-Interaction of solute&solvent: attractive form between solute particles and solvent particles. "solvation"or "hydration" (water=solvent)
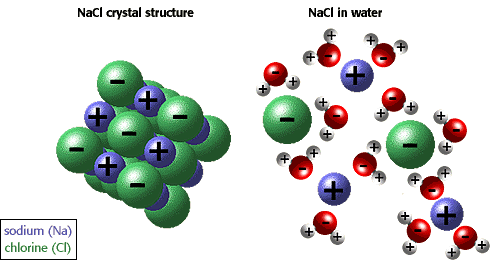
We had a brieft discussion about how like molecules disolve like molecules. That was the end of our discussion for todays class.
The NExt Scribe is Declan G
No comments:
Post a Comment