1.The first of these are Bonds.
2.The second are Non-Bonding pairs.
Both of these will affect the Structure of our molecule, but the only part of the structure we see are the bonded Pairs. This will make more sense whence you understand the Valence Shell Electron Pair Repulsion (VSEPR) rule. Under VSEPR electrons pairs in the outermost shells will attempt to go as far away from each other as possible. This means that although we don't look at the Non-Bonding electrons in the structure, they still are Under VSEPR and are effecting the shape of the electrons.
Although it is called Electron Pair Repulsion, we are not simply looking at the pairs, we are looking at areas of electron. I mean to say that if we were to encounter a double bond between two atoms, we would not expect the two pairs involved in the bonding to repel each other. No, these Double Bonds and triple bonds stick together.
In drawing the shape of Atoms we look at the number of Electron Areas around the Central Atom. A different structure is given based on how many electron Area's are around the central Atom and how many of these Areas are Bonds or Non-bonding Pairs. The Notes did a very good job of going through these types but because I do not have access to them, I'll go through it with you now.
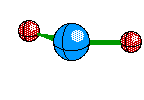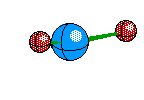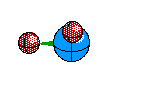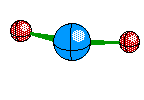
Linear is the structure given when their are only two Electron Areas surrounding the central atom and both are Bonds. The atoms are exactly 180 degrees from each other. This structure looks like this
Trigonal Planar this structure happens when the central atom has three electron Areas and all three are Bonds. The atoms still all fit on a plane and are all 120 degrees from eachother. This structure looks like so
Bent Molecule This happens when their are only two Bonds, like Linear, but the central atom also has A non-bounding pair. This causes the atoms to remain 120 degrees from eachother but we only see two of the atoms, and we see a bent structure like this
Tetrahedral Molecules get a little more complex because they now become three dimensional. When their are four electron areas, the atom seperates every atom by 109.5 degrees to look like so
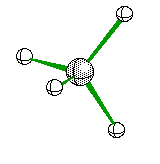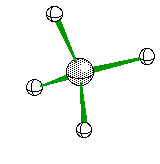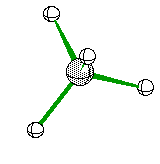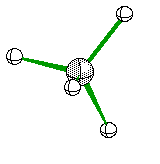
or when drawn on paper it looks something like
Triangular Pyramid when one of the four electron areas is a non-bonding pair it looks something like this
If two of the four electron ares are Non-Bonding then we see another Bent Structure, but in this case it is slightly more tight of an angle creating about a 104 degree bent.
Well there you have the atom structures that we have learned so far. No homework tonight but try to keep on track of upcoming webassigns. thank you, the next scribe will be
Takashi, have fun.
No comments:
Post a Comment