Reaction #1: CaC2 + 2H2O --> Ca(OH)2 + C2H2
Then we plugged C2H2 into a combustion reaction: (It is balanced..DUH) 2C2H2 + 5O2 --> 4CO2 + 2H2O
Mr. Lieberman then posed to the question of: If .60 grams of CaC2 reacts, how many grams of H2O will form? ( Use the balanced combustion equation above)
The converstions look like this:
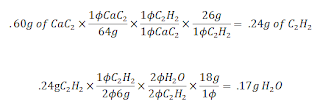
In the end you end up with .17 moles of H2O. Soon after this we witnessed the astonishing demo of Evil KinMOLble! This unlucky or lucky, (I'm not sure what was going through his head..... probably AAAAAAAAAAAAAAAHHHHHHHHHHHHH!!!!!!!!!!!!!) It was the projectilation of Mr. Mole from Mr. Lieberman's Mole Cannon using the combustion. The combustion was caused by the ignition of Acetylene from the reaction of H2O and CaC2(I think not sure on this). After that first demo, we took our short little quiz on Stoichiometry. Lastly, as a conclusion to our class period, Mr. Lieberman armed the launcher once again and fired it into the ChemPhys classroom next door. Causing complete academic Armageddon in the science wing. (ok I exhaggerated a little). Check out the Video in the post underneith this.
The next scribe is Tom btw.
No comments:
Post a Comment