The molecular equation is just another name for a balanced equation, which we learned how to do several classes ago. It's important that your molecular equation is written correctly before going any further, so make sure you pay attention that everything is correctly balanced. If not, the whole process afterward will be incorrect. Next, Mr. Lieberman introduced two new terms: complete ionic equations and net ionic equations. The complete ionic equation is the balanced equation with all the strong electrolytes written in as separate ions. So, for example, to make this molecular equation:
KCl + AgNO₃ ➝ KNO₃ + AgCl
into it's complete ionic equation, you would find out the electrical charge of each of the elements except for the precipitate. So, it would look like this:
K+ + Cl- + Ag+ + 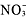 ➝ AgCl + K+ +
➝ AgCl + K+ + 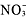
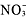 ➝ AgCl + K+ +
➝ AgCl + K+ + 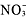
Every element shows it's electrical charge except AgCl since that is the precipitate [solid] formed. Lastly, Mr. Lieberman talked about a net ionic equation, which only includes the ions that undergo a change. That means that the spectators [ions that do not undergo a change] are not included. So, looking at the complete ionic equation above, the net ionic equation would be:
Cl- + Ag+ ➝ AgCl (s)
And that was basically all we did for the notes! After that, we had the rest of class to work on the Net Ionic Equation Worksheet. If you don't have it or have lost it, you can download it here and if you would like to check your answers, click here. Mr. Lieberman also mentioned that you should have done the Dissolving worksheet we got a couple days ago before attempting this one. The answers for that worksheet is here.
So that was Friday's class! Homework for Monday is the Chapter 4.2 Webassign and the Pre-Lab for the Double Replacement Reactions and Solubility Lab [to download, click here]. Also for the lab, make sure you create a data table! It's supposed to be a 6 x 8 grid with the rows and columns labeled with the anion solutions and cation solutions respectively.
Oh, and the scribe for Monday will be Declan G. Good luck! :]
No comments:
Post a Comment